Consortium
Who, What & Why
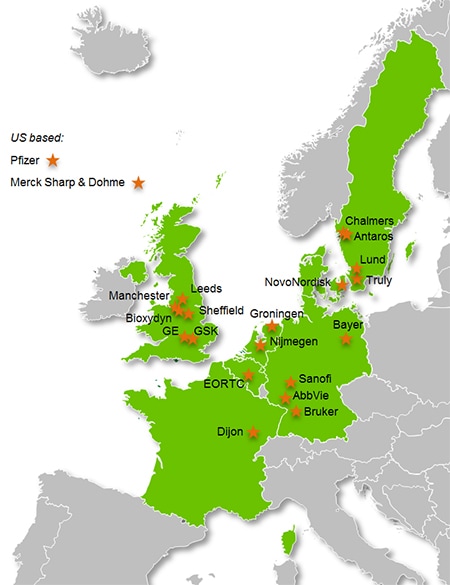
THE CONSORTIUM
- International non-profit organisation:
EORTC (Coordinator) - Pharma Companies:
Abbvie, Bayer (Lead), GSK (Co-lead), Merck Sharp & Dohme, Novo Nordisk, Pfizer, Sanofi - Imaging Vendors:
Bruker, GE Healthcare - SMEs:
Antaros, Bioxydyn, Truly - Universities:
Chalmers, Dijon, Groningen, Leeds, Lund, Manchester, Nijmegen, Sheffield
- Liver-bile transporter assessment (MRP2 & BSEP) by MRI & potentially PET
- Drug Induced Lung Toxicity Assessment by CT, 1H/129Xe-MRI, PET
- Bio-distribution of biologics by PET and MALDI-MSI
Liver
- Detailed kinetic profiling of liver transporters responsible for gadoxetate excretion
- Ensure translatability of gadoxetate DCE-MRI between imaging platforms (across vendors & pre-clinical vs. clinical platforms)
- Refine gadoxetate DCE-MRI in animals, analysing inhibitors for OATPs MRP2 and BSEP
- Optimize MRI techniques for improved usability in animals and humans
- Analyse gadoxetate DCE-MRI in healthy volunteers & in pruritis /cholestasis patients
- Investigate further BSEP specific imaging probes (PET & MRI)
- → Read more on imaging based liver transporter assessment
Lung
- clinical studies assessing DIILD: cancer patients & rheumatology patients to characterize lung issues by CT and 1H/129Xe MRI
- Use optimized CT & MRI methods in rats in order to refine animal models
- Characterize imaging biomarkers in rat models exhibiting different aspects of ILD
- Verify multicenter reproducibility
- Translate promising novel imaging biomarker
- → Read more on DIILD assessment by imaging
Biologics-PET
- Optimize 89Zr chelate & protein labeling strategy for improved protein PET
- Investigate Ab and peptide labeling with 89Zr in vitro and in vivo, compare to auto-radiography, validate with MALDI imaging methods (MSI)
- Validate 89Zr-Ab in patients against pre-clinical findings
- Refine IL2-PET labeling and utilization for assessment of downstream immunological effects in vitro & in vivo
- Use refined IL2 approach in patients receiving therapy
- → Read more on PET imaging for biologics
